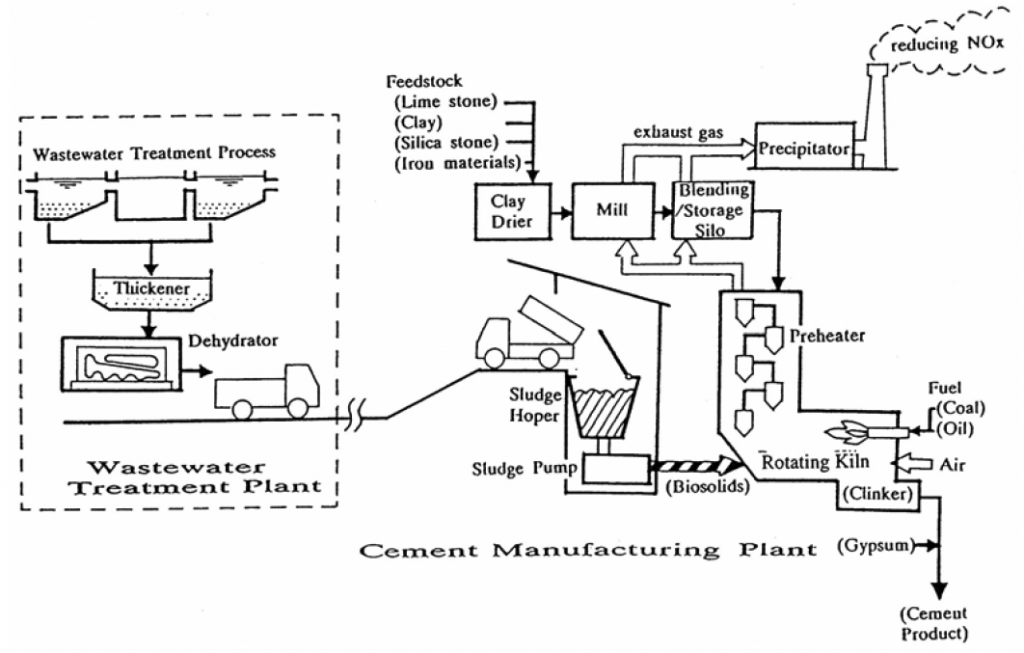
Calcium silicate Hydrates(C-H-S)
During the coarse of reaction of C3S and C2S of both side of water are evolved.
2C3S +6H----------->C3S2H3+3Ca(OH)2
2C3S +4H----------->C3S2H3+Ca(OH)2
C3S as compared to C2S produces less quantity of calcium silicate Hydrate and more quantity of Ca(OH)2 in the hydration process.
Ca(OH)2 is not desirable product in the concrete mass because it makes the concrete porous as C3S readily reacts in to water and produces more heat of hydration and it is responsible for early strength of concrete.
C3S Hydrates rather slowly and is responsible for later strength of concrete and produces less heat of hydration.The quality and density is an of calcium silicate hydrates produces C2S is better than that produces in the hydration of C2S.
Calcium Aluminium Hydrates (C-A-H)
Due to hydration of C3 A a calcium aluminate system of the form CaO Al2O3H2O. The Cubic Compound C3 A H6 is only stable compound formed and remains stable up to 225 C .The Reaction of C3 A with water is very fast and this may lead flash set.The hydrated Aluminate do not contribute any thing to strength of the cement post and it hydrates very fast and it may contribute little early strength.
On hydration of C4AF ,it is believed system of the form CaO Fe2O3 H2O and this hydrated calcium Ferrite and is compared to be more stable.This Product doesn't contribute any thing to the strength.
The hydrates of C4AF shows a compartively higher resistance to sulphate attack then hydrates of C2HA.
Calcium Hydroxide:
The other products of hydration of C3S and C2S is Ca(OH)2. The solution contributes 20-25% of the volume of the solids in the hydrate paste.The lack of durability of he concrete is on the account of Ca(OH)2 .It reacts with sulphates present in water or soil to form calcium sulphate which is further reacts with C3 A and cause detotration of concrete in which reaction with gypsum are
C3 A+32H +3CaSO4---------------->C6AS3H2
C3 A+18H+CaSO4-------------------> C4ASH